CLB’s N-1 perfusion manufacturing process
 By Travis Pysar, Chief Operating Officer, Cheerland Biotechnology
By Travis Pysar, Chief Operating Officer, Cheerland Biotechnology
Perfusion Processes
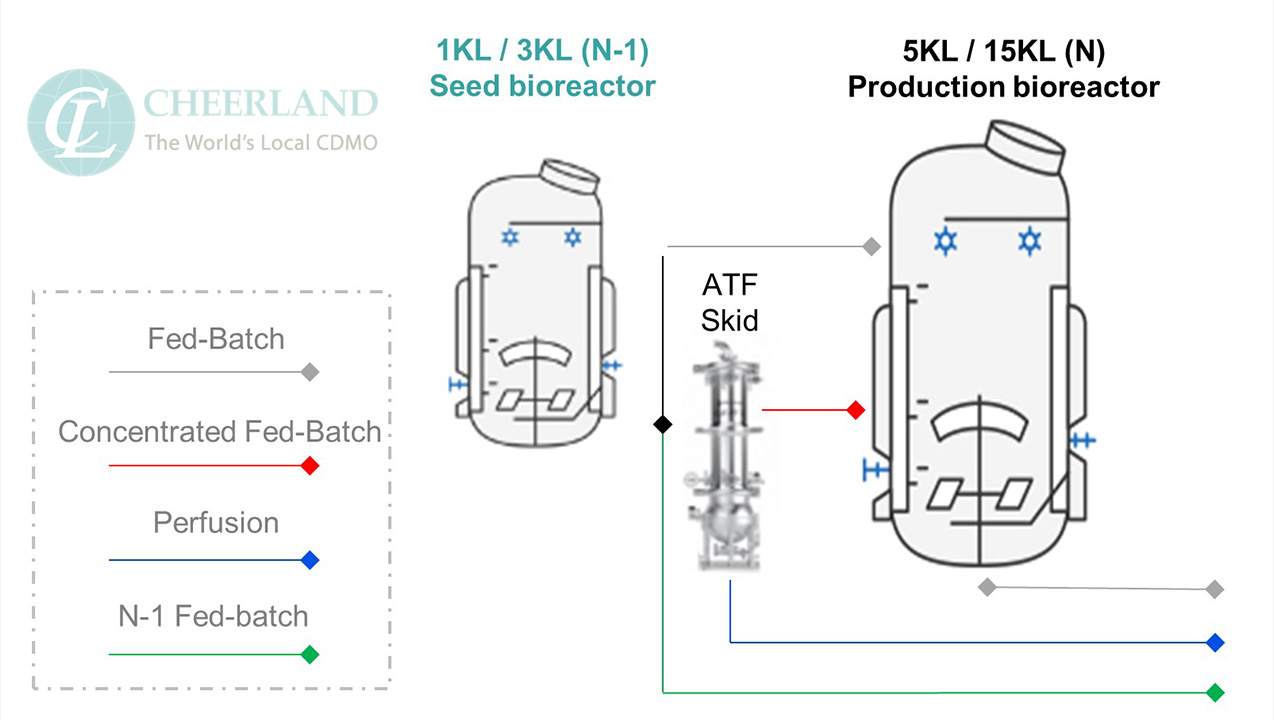
The N-1 bioreactor can be utilized as the production bioreactor for a perfusion process or continuous manufacturing process. Perfusion processes rely on smaller bioreactor volumes where the product is harvested on a continual basis and the harvested material is replaced with fresh media. Utilizing the smaller N-1 bioreactor as the perfusion production bioreactor decreased the media required to maintain the cell culture, thereby reducing the media prep vessel size requirements and reducing the cost of the media. Perfusion processes can easily achieve a 10 g/L titer over a typical 14-day process, thereby increasing the output compared to a standard fed-batch process.
CLB has incorporated a direct transfer from the N-1 bioreactor to facilitate a perfusion process or allow for a smaller production bioreactor size for fed-batch process.
Process Intensification
The N-1 bioreactor can also be utilized to intensify the cell culture density in the seed train prior to inoculating the production bioreactor. Similar to a perfusion process, the media is continually replaced with fresh media to support high cell densities in the N-1 bioreactor. The production bioreactor is then inoculated with a high seed density to promote higher titers in the production bioreactor. This process intensification can yield titers from 8-10 g/L in the production bioreactor.
Flexibility
CLB is committed to meeting the needs of all of our clients, whether that is through a standard fed-batch, an N-1 perfusion batch, a N-1 fed-batch, or an N-1 process intensification. Our state of the art facilities offer the most flexibility and the highest productivity.









