CLD Services
- Vector construction
- Cell line development – CHO-K1, CHO-S
- Cell Bank Creation and Characterization
- Construction of stable cell lines
- Transient sample expression
DS – Downstream Services
- Platform Purification Process Development
- Customer Process Transfers / Development
- Purification Viral Clearance Studies
DS – Upstream Services
- Cell Culture Process Development
- Customer Process Transfers
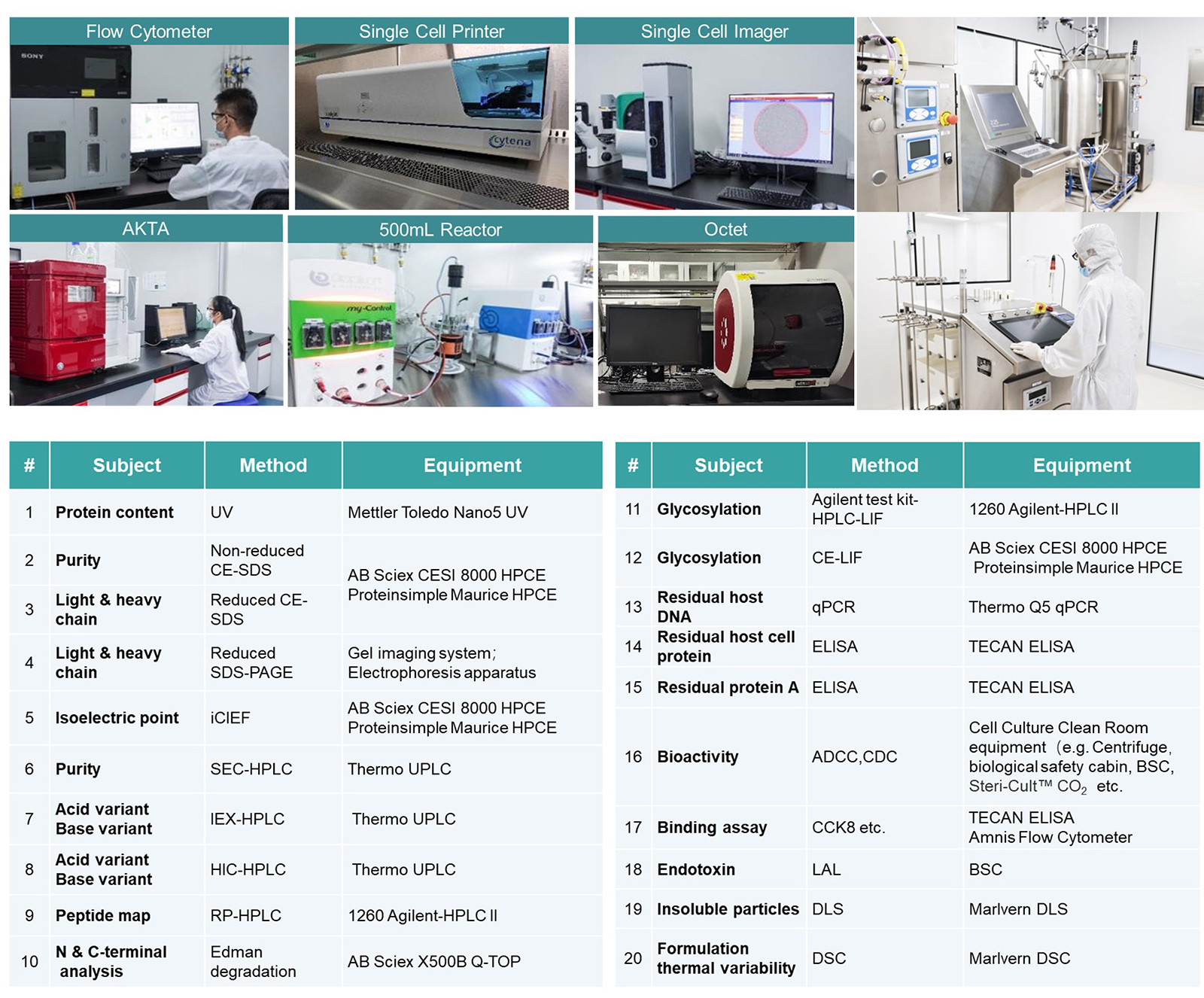
DS – Analytical Development
- Platform Analytical Methods
- Method Development / Transfer / Validation
- Protein Characterization
- Stability Testing
cGMP Manufacturing
- Dedicated single-use production lines
- ~ 24 batches per year
- GMP ready